A) Reactions are favored (i.e. have a motive) if they lead to formation of a weaker acid and/or weaker base.
B) Checking pKa values can predict if a reaction has a motive even if there are other steps besides a proton transfer.
C) Recall that the conjugate base of a stronger acid (lower pKa) is a weaker base.
D) Check the pK's of the conjugate acid of the bases on either side of the equation. Lower pKA value corresponds to stronger acid of the conjugate acid, and thus weaker conjugate base. The base with a stronger conjugate acid (lower pKa value) will be the weaker base and will be favored at equilibrium.
E) Another way to look at it is that the base that is favored at equilibrium is the one that has the more stabilizied anion, i.e. the one with the charge spread around more (electronegative) atoms.
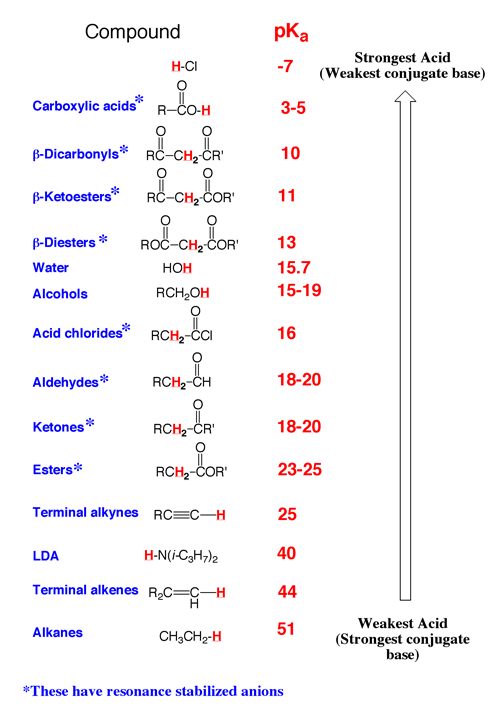
F) Above is a pKa table that we will refer to often.